Sec. 2.6 Origin of life [1]
2.6.1 Formation of cell membranesThe solar wind H+ is CO2
Fig. 2.6.1. Synthesis of hydrocarbon molecules in the upper sky
The high-speed H+ of the solar wind collides with the CO2 of the atmosphere above the proto-Earth, causing
H2O and CH4 and so on to make hydrocarbons. Hydrocarbons with low boiling temperatures
remain in the sky and continue the polymerization reaction. In the polymerization
reaction, long-chain hydrocarbons are synthesized by the repulsion of H+ bound to C.
Current biological cell membranes are mainly composed of the hydrocarbon molecules C16H34 or C18H38. C16H34 has a melting point of 20°C and a boiling point of 300°C. C18H38has a melting point of 27.8°C and a boiling point of 316.15°C. Both are liquids in the terrestrial environment of the primitive Earth.
Hydrocarbons have a light specific gravity and are hydrophobic, and float as an oil film on the surface of the water.
Fatty acids are formed by oxygen binding to the tail of hydrocarbons floating on the surface of the water to form a carboxyl group.
Since this molecule does not pass through the membrane of the water surface, a cell membrane was formed outside the hydrophilic part.
2.6.2 Liquid water with a spiral-type lattice structure
The light reflected on the surface of the water glitters, but when you
look at the surface of the water with polarized glasses, the reflected
light disappears. It involves the spiral structure of the water. Water
(H2O) is a structure of one huge molecule linked by hydrogen bonds. The three-dimensional
structure changes frequently. The lowest-energy structure of the lattice
structure, which consists of tetrahedral molecules is a α-quartz type structure,
this structure is a spiral structure with a planar structure as the base.
The surface of the water is planar, and water molecules instantaneously
create a spiral arrangement structure. This helical structure has open
shafts in which elements of long chain molecules may penetrate. The long
chain hydrocarbon molecules support to form the cell membrane.

Fig. 2. 6.2 H2O molecules surrounding a vacant “shaft” of the spiral structure in liquid
water.
2.6.3 Concurrent synthesis of proteins and nucleic acids in cell the membrane
All living things on Earth are composed of proteins consisting of amino acids with chirality of type L. And chirality has D-type sugar (a building block of DNA). Because carbohydrates {Cx(H2O)y} can be formed on the surface of hydrocarbons suspended on the surface
of water, long chains of polymeric molecules consisting of monosaccharides
bonded by glycosidic bonds are formed from oil films. A long-chain polymeric
carbohydrate thread consisting of its monosaccharide units is added to
the region adjacent to the individual amino acids of the chirality L shape
that invade when making proteins. Nucleic acids of sugar molecules are
produced. In other words, the amino acid units of messenger RNA are synthesized
in the opposite direction around amino acids bound to synthesize proteins
and invade the interior.
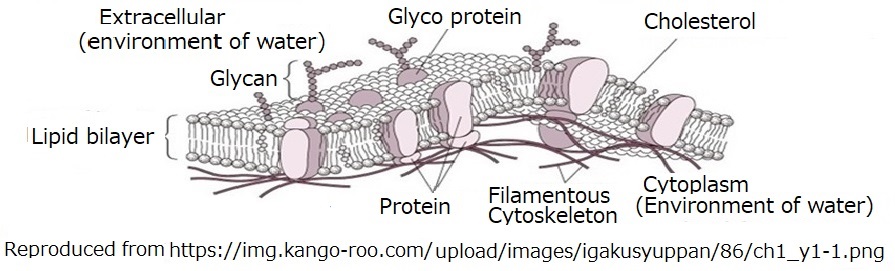
Fig. 2. 6. 3 Concurrent syntheses of filamentous molecules of proteins
and nucleic acids in cell membranes
2.6.4 How to control the synthesis of protein in a creature?
DNA, which inherits information on protein synthesis, is composed of two helical structures with different chirality’s facing each other. One is D-type transport RNA (t-RNA), and the other is L-type messenger RNA (m-RNA) to synthesize proteins. Here, when amino acids physically bind to m-RNA, amino acid sequences are incorporated at random times, making accurate synthesis impossible. Each amino acid unit of the m-RNA sequence is a codon of the genetic code and is matched against the anticodon of t-RNA carrying the amino acid. When the codon and anticodon match, the amino acids of t-RNA are linked to the m-RNA sequence to synthesize the protein. By the relationship between the key and lock of the two genetic codes, the sequence of amino acid units of m-RNA and the amino acid progression of protein molecules are geared meshed at one point. Proceed with replication only if it is. Here, molecules with different chirality’s are in opposite rotations.
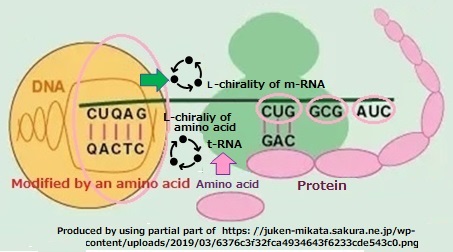
Fig. 2.6.4 The amino acid unit sequence of m-RNA and the amino acid progression of protein molecules replicate when the genetic code meshes.
2.6.5 Configuration of DNA
Since m-RNA has a short lifespan, the helix sugar of type D m-RNA forms
DNA in a stable molecular region by dehydration binding. When this double
helix structure is twisted, the L-type molecular string approaches or separates
from the D-type molecular column depending on the direction of rotation.
Through this mechanism, proteins with various properties were synthesized.
In order for these proteins to function well, it is necessary to change
the system accordingly. In this way, living organisms began to engage in
vital activity.
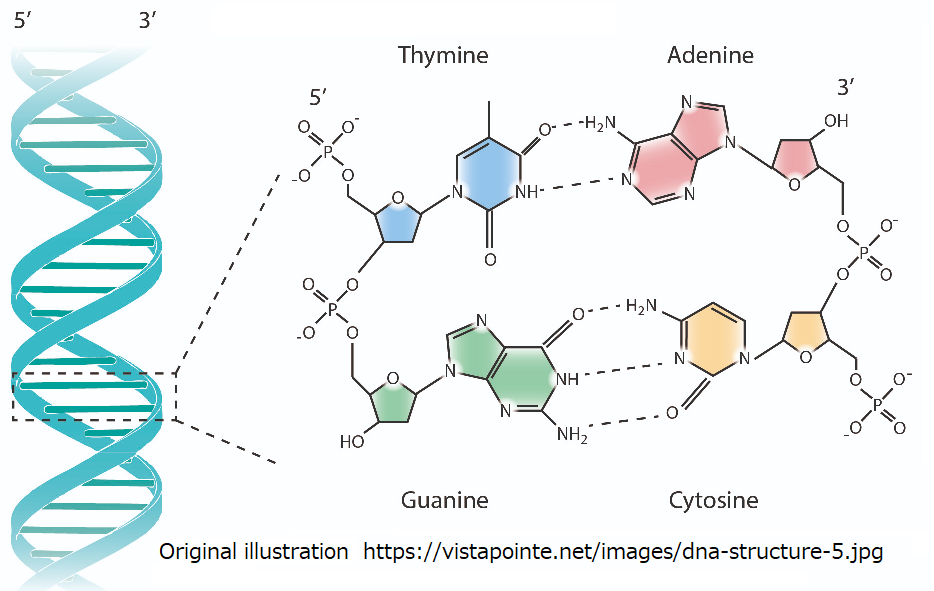
Fig.2.6.5 Structure of DNA that constitutes with double helix structure.
[Reference]
[1] S. Karasawa, “Origin of life in the water of the Earth”, The journal of Geology, Earth & Marine Science, [ISSN: 2769-0571] , 2023.
to index page -2.6-